Abstract
Background: Patients ≥55 years of age with active, relapsed or refractory acute myeloid leukemia (R/R AML) who have failed standard induction therapies most often receive salvage therapy with a wide variety of agents, but very few achieve remission. These patients do not routinely undergo allogeneic hematopoietic cell transplantation (HCT) due to lack of efficacy using a standard HCT approach. The SIERRA trial is a prospective, randomized, phase 3, open-label, ongoing multicenter trial designed to address this significant unmet need in R/R AML. This trial compares Iomab-B, an 131I-radiolabeled anti-CD45 antibody as targeted conditioning prior to HCT against standard conventional care therapies with an accrual goal of 150 patients. We have performed a preliminary safety analysis, validating the initial feasibility of this multi-center trial.
Methods: Eligible patients are ≥ 55 years of age with active, R/R AML, adequate organ function, and related/unrelated matched donor. Patients are randomized (1:1 ratio) to receive dosimetry directed Iomab-B followed 12 days later by HCT with fludarabine 30 mg/m2 x 3 days and 2 Gy of total body irradiation as transplant conditioning, or to a Conventional Care (CC) arm. Patients randomized to CC, may receive investigator's choice of salvage induction chemotherapy including approved targeted agents as well as Venetoclax (in combination with a hypomethylating agent) and proceed to standard HCT if they achieve complete remission (CR). If patients do not achieve CR, the study allows optional cross-over to the Iomab-B arm, evaluated between days 28-42 after CC therapy. The primary efficacy endpoint for the study is durable complete remission (dCR) of 6 months. The secondary efficacy objective is to evaluate overall survival at 1 year.
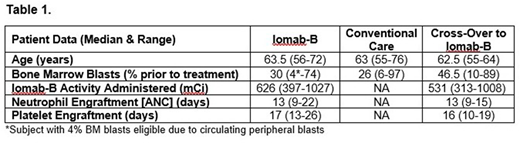
Results: Forty patients have been enrolled to date and data are available for 38 patients (19 = Iomab-B, 19 = CC) as of July 5th, 2018. The median age was 63 years (range: 55-76). All patients had active disease at the time of enrollment with median bone marrow (BM) blasts of 30% in the Iomab-B arm and 47% in the crossover arm prior to receiving Iomab-B (Table 1). Of the patients in the CC arm, 88% did not achieve a CR and 65% of those crossed-over to receive Iomab-B. The most common reason preventing crossover was declining performance status. Time to HCT was not increased in the crossover patients receiving Iomab-B compared to those that underwent a standard of care transplant after achieving CR (median 65 vs 75 days), while the Iomab-B arm patients were transplanted at a median of 27 days after randomization. Iomab-B therapy was well-tolerated with no grade 3 or 4 infusion-related reactions reported. The median activity of Iomab-B was 626 mCi (range: 397-1027). All patients randomized to Iomab-B (N=13) engrafted, with median days to engraftment of ANC at 13 (range: 9-22) and platelets at 17 (range: 13-26). All patients who received Iomab-B after crossover (N=9) also engrafted with time to engraftment similar to patients initially randomized to the Iomab-B arm (Table 1). Donor chimerism (≥95%), within 100 days after HCT, was found in 9 of 10 patients in the Iomab-B arm and 8 of 9 patients in those that crossed over to receive Iomab-B, with two patient exceptions having mixed donor chimerism at day 28. A preliminary safety analysis for all randomized patients on either study arm showed the most frequent non-hematologic adverse events ≥ grade 3 occurring in >10% patients were febrile neutropenia (34.2%), stomatitis (15.8%), malnutrition (13.2%), epistaxis, sepsis, hypotension, hypobilirubinemia and fatigue (all 10.5%). There were no Iomab-B-related deaths.
Interpretation: Preliminary safety analysis demonstrates the feasibility of delivering targeted conditioning with Iomab- B for HCT in R/R AML patients who have active disease and a high BM blast burden (median: ≥ 30%) prior to transplantation. All patients who received Iomab-B, including those crossed over after failing to achieve CR on CC salvage therapy, engrafted ANC within 13 days, despite active disease prior to transplant. In addition, the time to HCT from randomization was not increased in patients receiving Iomab-B after cross-over compared to those who underwent standard of care HCT after achieving CR on the CC arm. This study is currently enrolling and for full study details see www.sierratrial.com or clinicaltrials.gov (NCT02665065).
Tomlinson:Foundation Medicine: Consultancy. Foran:Xencor, Inc.: Research Funding; Agios: Research Funding. Chen:Bellicum Pharmaceuticals: Research Funding. Levy:Takeda (Millennium Pharmaceuticals, Inc.): Consultancy. Lazarus:Pluristem Ltd.: Consultancy. Berger:Actinium Pharmaceuticals: Employment, Equity Ownership. Reddy:Actinium Pharmaceuticals: Employment, Equity Ownership; Pharmacyclics: Employment. Pagel:Pharmacyclics, an AbbVie Company: Consultancy; Gilead: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract